For people with plaque psoriasis (6+) or ERA (4+) or PsA (2+)

COSENTYX® (secukinumab) is approved for adults and children 6 years and older with moderate to severe plaque psoriasis.
Pediatric psoriasis isn't just a skin condition—it's a long-lasting autoimmune disease that may be hereditary. When you have psoriasis, your immune system is overactive and can cause inflammation throughout the body. If your child has been struggling to manage their skin symptoms with creams, lotions, ointments, or light therapy, you should consider COSENTYX, which works to clear skin from the inside out.
In a clinical trial, most children aged 6-18 years with severe plaque psoriasis taking COSENTYX 75 mg or 150 mg† were clear or almost clear at 12 weeks.
For those taking 75 mg (less than 110 lbs):
32% were clear or almost clear
Approximately 6 out of 10 saw at least 75% skin clearance
Approximately 4 out of 10 saw at least 90% skin clearance
For those taking 150 mg (110 lbs or more):
81% were clear or almost clear
Approximately 9 out of 10 saw at least 75% skin clearance
Approximately 8 out of 10 saw at least 90% skin clearance
†Dosage strength based on child's weight.
For more on treating psoriasis in children, download our brochure, Treating Kids and Teens With COSENTYX.
How does COSENTYX work?
COSENTYX is a biologic treatment for plaque psoriasis in children and adults that works from the inside out. COSENTYX is designed to target and block IL-17A, which is believed to play a role in inflammation that causes the psoriasis plaques your child is seeing. Did you know an overactive immune system is like an overactive tennis ball machine? Watch this video to see how taking COSENTYX may help.

Patient portrayal of how COSENTYX works.
Experience matters
When you’re considering COSENTYX as a pediatric plaque psoriasis treatment, you should know that doctors have been prescribing COSENTYX for the treatment of plaque psoriasis in adults since 2015.
Over 5.2 million prescriptions of COSENTYX have been filled‡ in the United States§
COSENTYX is FDA approved to treat a number of autoimmune diseases, such as adult and pediatric plaque psoriasis, adult and pediatric active psoriatic arthritis, adult and pediatric enthesitis-related arthritis, adult active ankylosing spondylitis, and adult active non-radiographic axial spondyloarthritis
COSENTYX has been studied for more than 17 years in dozens of clinical trials‡
‡Across all indications combined.
§As of December 2024.

Individual results may vary. LauraLee was compensated for her time.
"I've had psoriasis since I was 16. Dealing with it as a teenager was hard, 'cuz kids can be so mean. I really wish COSENTYX had been around when I was younger."
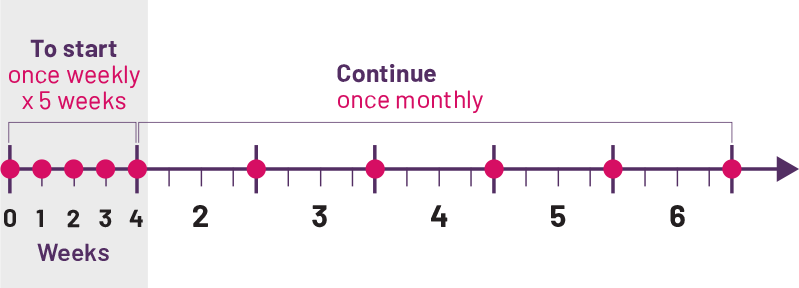
Convenient once-a-month dosing¶
Your child's doctor will prescribe the dose of COSENTYX that's right for your child, guided by your child’s weight. To start, your child will take COSENTYX once a week for 5 weeks. Then, just once a month for maintenance. This can mean fewer interruptions in both your and your child's daily routines.
If your child weighs less than 110 lbs, the recommended dose of COSENTYX is 75 mg. If your child weighs more than 110 lbs, the recommended dose is 150 mg.
If the prescribed dose of COSENTYX is 75 mg, you must give 1 injection of the COSENTYX 75-mg prefilled syringe for each dose
If the prescribed dose of COSENTYX is 150 mg, you must give 1 injection of the COSENTYX 150-mg Sensoready® Pen or 150-mg prefilled syringe for each dose
¶Monthly dose equals 1 dose every 4 weeks.
How to use the Prefilled Syringe
Watch How to Use the COSENTYX Prefilled Syringe (75 mg & 150 mg) to learn how to give a once-monthly injection, with helpful tips for parents and caregivers.
How to use the Sensoready Pen
Watch How to Use the COSENTYX Sensoready Pen (150 mg) to learn how to give a once-monthly injection, with helpful tips for parents and caregivers.
Get dedicated personal support and savings options for COSENTYX right from the start.
Sign up for COSENTYX® Connect, a free, customized program for those considering or taking COSENTYX. You'll get services and support to help both you and your child—including injection resources, savings options like a pay as little as $0 co-pay*, if privately insured and otherwise eligible, and a dedicated COSENTYX® Connect Team Member.