For people 2 years of age and older with active psoriatic arthritis

HOW TO TAKE COSENTYX® (secukinumab)
Whether it's self-injection or IV infusion,† your doctor can help you choose what's right for you.
Talk to your doctor. Together you can decide between self-injection at home or IV infusion in a medical office.
†For adults.
Self-Injection
Convenient once-a-month self-dosing with no routine lab monitoring
Taking COSENTYX once a month for your psoriatic arthritis (PsA), with no routine lab monitoring during treatment,† helps you have fewer interruptions in your daily routine and can help provide real relief from multiple symptoms of PsA.
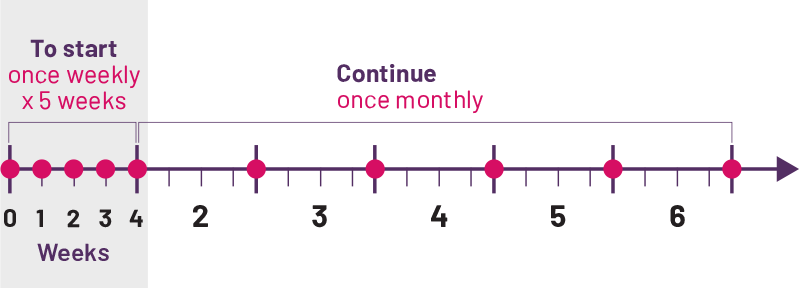
To start, your doctor will either prescribe 5 weekly doses or just 1 dose once a month, based on what's right for you. After that, you only need to take COSENTYX once a month.‡ The recommended dose is 150 mg, which is taken as 1 injection under the skin.
If you continue to have PsA symptoms, your rheumatologist may prescribe 300 mg (taken as two 150-mg injections).
If you have PsA but also have moderate to severe plaque psoriasis, your rheumatologist may prescribe 300 mg (taken as two 150-mg injections).
COSENTYX is injected with the Sensoready® Pen or a prefilled syringe.
†Before starting, get checked for tuberculosis.
‡Monthly dose equals 1 dose every 4 weeks.
If you're taking the 150-mg dose and you continue to experience some PsA symptoms, your rheumatologist may increase your dose to 300 mg. Do not try to inject COSENTYX yourself. Your healthcare provider should show you how to inject COSENTYX before using it for the first time.
Learn more about our devices and watch step-by-step videos on how to take COSENTYX.
The COSENTYX Sensoready Pen is designed for comfortable use:
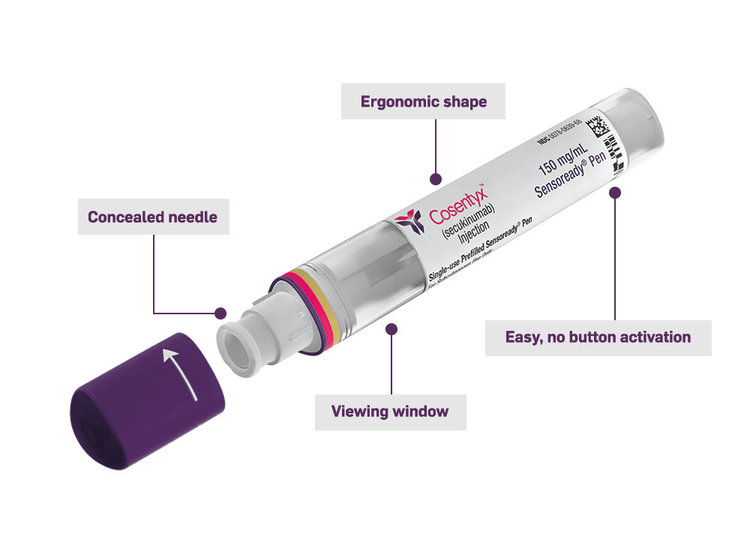
Concealed needle
You can self-inject safely and confidently
Viewing window
The green indicator assures you that your injection has been completely delivered
Easy, no-button activation
Automatic 2-click technology allows you to hear when your dose starts and finishes
Ergonomic shape
Designed to be easy to hold and easy to use
Learn more about how to use the Sensoready Pen and about COSENTYX in the prefilled syringe.
Find out why people are using the COSENTYX Sensoready Pen:
More than 90% of adults reported no pain or reaction during or after the injection.§
Nearly 9 out of 10 adults reported being satisfied or very satisfied with self-injection using the Sensoready Pen.§
Download this handy brochure with instructions and tips so you have them whenever you need them.
§In a study of 37 adult patients with active PsA.
Very few adults in a PsA clinical trial (1.6%) experienced any injection site reactions with COSENTYX||
- ||Results are from clinical trials at Week 16.
"What I like about the Sensoready Pen is that it's like...so easy to use."

Individual results may vary. Joni was compensated for her time.
If self-injection isn’t an option for you
Ask your doctor about COSENTYX IV infusion. A 30-minute infusion at the doctor's office or infusion center.
IV Infusion
COSENTYX is available through IV infusion
COSENTYX offers an additional treatment delivery option based on your weight through a monthly, 30-minute infusion. Plus, each infusion is administered by a healthcare provider at an infusion center, so you have support available to you during the process.
COSENTYX does not require any medications (such as antihistamines) to be administered before or after your infusion.
What to expect with COSENTYX taken through an IV
You will be given COSENTYX by a healthcare provider through a needle placed in your vein (infusion). It takes about 30 minutes to give you the full dose of COSENTYX.
Your healthcare provider will dose COSENTYX based on your weight.
Your healthcare provider will tell you how often you should receive COSENTYX.
If you miss an appointment to receive COSENTYX, make another appointment as soon as possible.
“When I transitioned from self-injection to IV infusion, it was a very smooth transition."
- Carolyn, Real Patient
Preparing for your COSENTYX infusion
Talk to your healthcare provider about what to expect, as each infusion center may be different.
Here are a few considerations when preparing for your infusion.
Before arriving at the infusion center:
Hydrate well
Dress comfortably
Bring something to entertain yourself
During your infusion, your healthcare provider may:
Check your vital signs
Ask you some questions
Answer any questions you may have
Once your infusion is complete:
Schedule your next infusion
If you miss an appointment to receive COSENTYX, make another appointment as soon as possible
"The infusions take 30 minutes. I'll often read a book while I'm there.”
—Carolyn, Real Patient
During your COSENTYX infusion
Your 30-minute infusion will be delivered while you remain seated. Others have found that it helps the time to go by faster by creating a routine or ritual that's unique to their experience. Here are a few ideas:
Listen to a favorite playlist or podcast
Read a book
Scroll through social media
Play a game
Catch up on emails
Infusing Relief with Carolyn
Carolyn, an art teacher and grandmother of six, is passionate about inspiring her students and spending time with her family. Watch how she found relief from years of pain and stiffness with COSENTYX through IV infusion, helping her keep doing what she loves most.