For people 6+ with moderate to severe plaque psoriasis

JUST ONE DOSE A MONTH
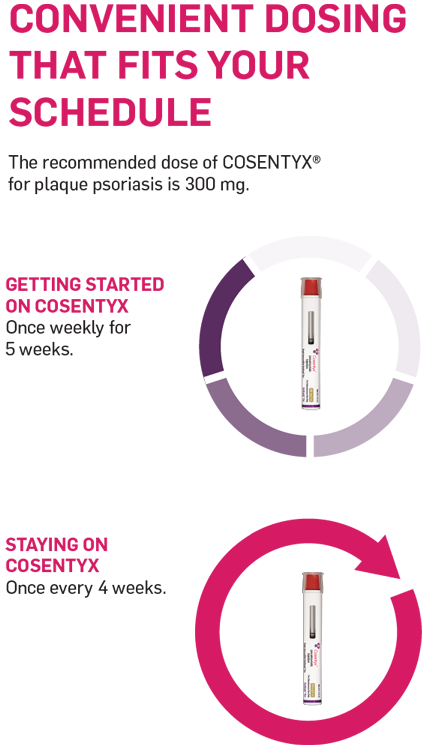
Convenient once-a-month dosing†
The recommended dose of COSENTYX® (secukinumab) for moderate-severe plaque psoriasis is 300 mg.† To start, your doctor will prescribe 5 weekly loading doses. To continue after that, you only need to take COSENTYX once a month for maintenance, which means fewer interruptions in your daily routine.
†Each 300-mg dose is given as 2 injections of 150 mg under the skin. For some people, a dose of 150 mg may be acceptable.
COSENTYX is injected with the Sensoready® Pen, UnoReady® Pen, or a prefilled syringe. Your healthcare provider should show you how to inject COSENTYX before using it for the first time.
Learn more about our devices and watch step-by-step videos on how to take COSENTYX.
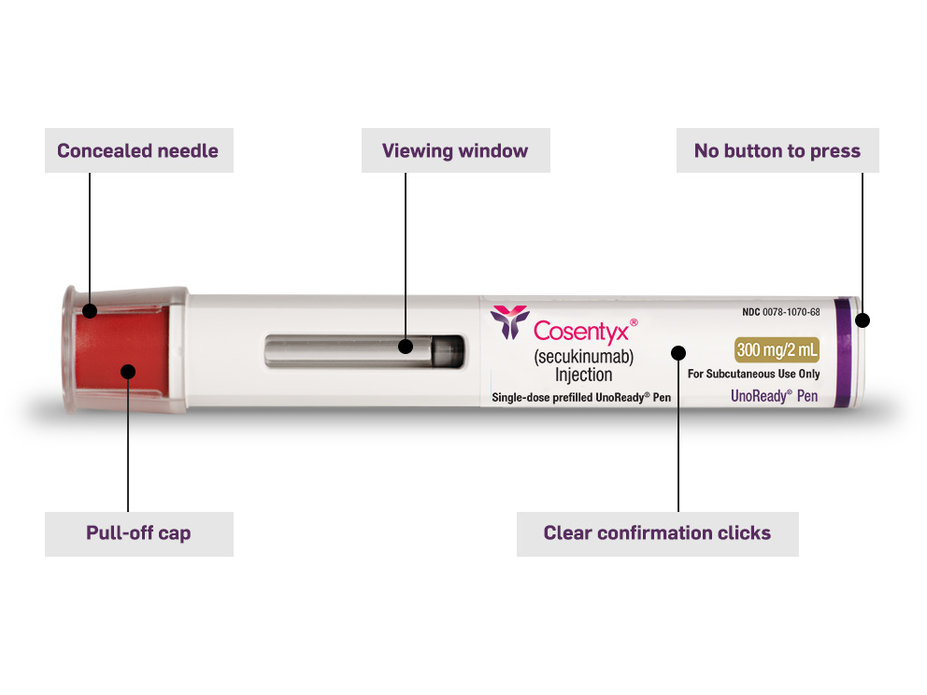
The COSENTYX UnoReady® Pen is designed for comfortable use
The COSENTYX UnoReady Pen has a positive satisfaction rating up to 100%.‡
The UnoReady Pen is a single, 300-mg injection
Design is convenient and easy to use
The needle is hidden
The cap is not made with natural rubber latex
The majority of people experience no pain with the injection§
Download and print the step-by-step Instructions for Use so you can have them handy whenever you need them. For more information about using the UnoReady Pen or the Sensoready® Pen, download and print the Quick Tips Brochure.
‡Based on a study in which all 37 participants using the UnoReady Pen reported being satisfied or very satisfied with the device at 28 weeks for patients with moderate to severe plaque psoriasis.
§Based on a study in which 30 of 38 participants using the UnoReady Pen reported no pain with the injection at 12 weeks.

Individual results may vary. Gary was compensated for his time.
“The UPS guy shows up. I put my medicine away. When it’s the day to take it, I put it in. It's practically painless. I'm good to go. I don't think about it for a month, and then I'm excited to call to reorder to have the UPS guy show up again.”