For people 2 years of age and older with active psoriatic arthritis

HOW DOES COSENTYX® (secukinumab) WORK?
Watch the video to see how COSENTYX works by targeting IL-17A, a molecule in the body that causes inflammation in people with psoriatic arthritis.†
Did you know an overactive immune system is like an overactive tennis ball machine? There are multiple molecules in the body that may play a role in causing inflammation. The IL-17A molecule is just one of them.
Patient portrayal of how COSENTYX works.
†The relationship between how COSENTYX works in the body and how it affects your symptoms is unknown.
How does COSENTYX work?
COSENTYX is a targeted biologic. While other treatments may have different ways of working, COSENTYX selectively blocks IL-17A, a protein that causes psoriatic arthritis symptoms that can lead to permanent joint damage. It’s the first and only treatment to offer both self-injection and IV infusion dosing options that target IL-17A.‡
What is a biologic?
A biologic is a protein-based medication that is made from living sources, and is taken by injection or infusion.
‡For adults.
If left untreated, psoriatic arthritis can cause permanent joint damage.
Psoriatic arthritis (PsA) symptoms can progress and your joints, such as your fingers or toes, can become permanently damaged. This can disrupt your ability to perform daily activities. COSENTYX is proven to help stop further joint damage.
As part of a large study on psoriatic arthritis, COSENTYX was proven to help stop further joint damage.§, ||, ¶
Relief you can see. On X-rays in clinical trials, 77% of people taking COSENTYX had no further joint damage at 6 months.#
§Based on data from X-rays of the hands, wrists, and feet at 6 months.
||Administered subcutaneously (under your skin).
¶All results shown in clinical trials using subcutaneous (SC) administration (injection under the skin). The FDA approval of the intravenous (IV) administration (injected into a vein) of COSENTYX is based on data showing that the amount of the medication in your body after it is given directly through IV was within the range that would be seen when given as SC.
#For adults taking the 300-mg dose of COSENTYX. 71% of patients taking the 150-mg dose had no further increase in joint damage at 6 months, compared with 68% of those not taking COSENTYX (placebo).

Individual results may vary. Darren was compensated for his time.
“I had a lot of swollen joints and psoriasis flakes all over. It was hard to get up in the morning and get ready. With COSENTYX, I started looking and feeling better in a short time. And since then, I’m doing the things I want to do with much less pain.”
GET RESULTS FAST THAT CAN LAST
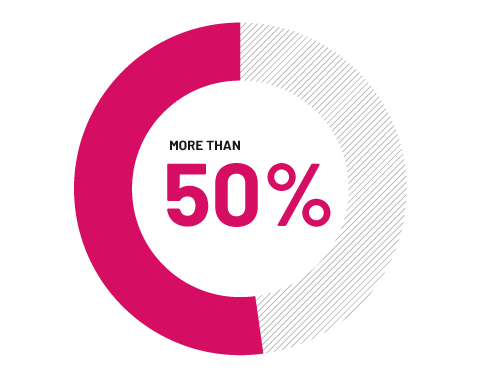
Complete reduction of swelling
In a clinical trial, more than half of adults with completely swollen fingers and toes (dactylitis) no longer had signs of swelling after 6 months of taking COSENTYX versus 15% for placebo.
