For people with plaque psoriasis (6+) or ERA (4+) or PsA (2+)

COSENTYX helps kids and teens with Enthesitis-Related Arthritis (ERA) and Juvenile Psoriatic Arthritis (JPsA), 2 forms of juvenile idiopathic arthritis (JIA), the most common juvenile arthritis (JA).
In a clinical trial of kids and teens with enthesitis-related arthritis (ERA) or juvenile psoriatic arthritis (JPsA) taking COSENTYX® (secukinumab):
Those with ERA had a 53% reduced risk of flares
Those with JPsA had an 85% reduced risk of flares
A flare is when your child's symptoms become worse without much improvement, and involves at least 2 joints.
For more on real relief with COSENTYX, download our brochure.
SYMPTOM IMPROVEMENT IN AS EARLY AS 12 WEEKS.
In a clinical trial of kids and teens taking COSENTYX, 6 key areas were observed. Most (91% with JPsA and 85% with ERA) showed at least a 30% improvement in 3 or more of the 6 key areas, with worsening in no more than 1 key area, after 12 weeks of treatment.
The doctor's assessment of disease activity
The parent's or child's assessment of overall well-being
Difficulty doing daily activities
Number of joints affected by active arthritis
Number of joints that are hard to move
Lab test that measures inflammation
COSENTYX is the only medicine FDA approved to treat 2 types of JIA: ERA and JPsA
JIA, an autoimmune inflammatory disease, is the most common form of Juvenile Arthritis (JA). There are 7 different types of JIA, and each one has its own symptoms.
UNDERSTAND ERA & JPSA, THE TWO MOST COMMON FORMS OF JA
ERA
Enthesitis-related arthritis (ERA) is a type of JIA that affects the tissue where the muscles, ligaments, or tendons meet the bone (entheses). Symptoms may include swelling, joint pain, and stiffness at the hips, knees, and feet. The fingers, elbows, pelvis, chest, and lower back can also be affected.
JPsA
Juvenile psoriatic arthritis (JPsA) is a type of JIA that may include symptoms of both arthritis and plaque psoriasis. Arthritis symptoms can show up before skin symptoms and may affect 1 or more joints, often in the wrists, ankles, fingers, or toes. Psoriasis can appear as a scaly rash behind the ears, on the eyelids, elbows, knees, belly button, or scalp.
COSENTYX has proven experience
When you’re considering COSENTYX as a treatment for your child’s ERA or JPsA, you should know that COSENTYX is the most prescribed medicine of its kind,† and is approved to treat pediatric plaque psoriasis.
Over 6 million prescriptions of COSENTYX have been filled† in the United States*
Well-studied safety profile since its launch in 2015
No blood tests or routine lab monitoring required during treatment with COSENTYX
COSENTYX has been studied extensively for more than 18 years in dozens of clinical trials across all indications. More clinical trials are ongoing as we stay committed to studying COSENTYX.
*Across all indications combined.
†As of November 2025.
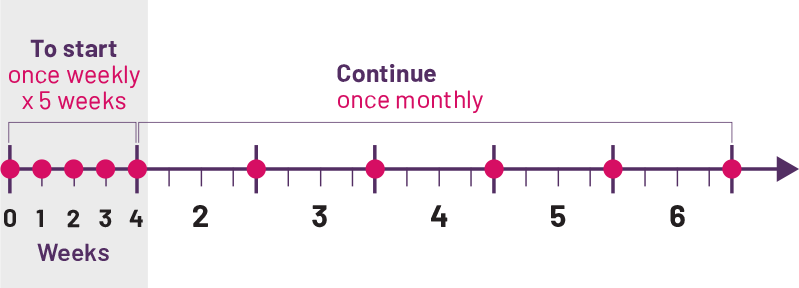
Convenient once-a-month dosing,‡ guided by your child’s weight
Your child’s doctor will prescribe the dose of COSENTYX that’s right for your child based on their weight. Your child will take COSENTYX once a week for 5 weeks, then just once a month afterward. This can mean fewer interruptions in both your and your child’s daily routines.
If your child weighs 33 lbs or more but less than 110 lbs, the recommended dose of COSENTYX is 75 mg. If your child weighs 110 lbs or more, the recommended dose is 150 mg.
COSENTYX requires just 1 injection for each dose (a prefilled syringe for a 75-mg dose or a prefilled syringe or Sensoready® Pen for a 150-mg dose).
‡Monthly dose equals 1 dose every 4 weeks.
How does COSENTYX work?
Having ERA or JPsA means your child has an overactive immune system. COSENTYX is a biologic treatment that works within the body's immune system to selectively target and block just the IL-17A molecule, which may play a role in causing inflammation throughout the body when it is overproduced. Blocking IL-17A may help reduce the inflammation causing the joint pain and stiffness your child feels.
What is a biologic?
A biologic is a protein-based medication that is made from living sources and is taken by injection or infusion.
COSENTYX® Connect is here for you throughout your child's treatment.
COSENTYX® Connect is a free support program for people taking or considering COSENTYX. Our goal is to make the COSENTYX experience as easy, affordable, and convenient as possible for you and your child. Sign up now and you'll have access to a full range of services and support, like a COSENTYX® Connect Team Member, co-pay,* if privately insured and otherwise eligible, and injection resources.